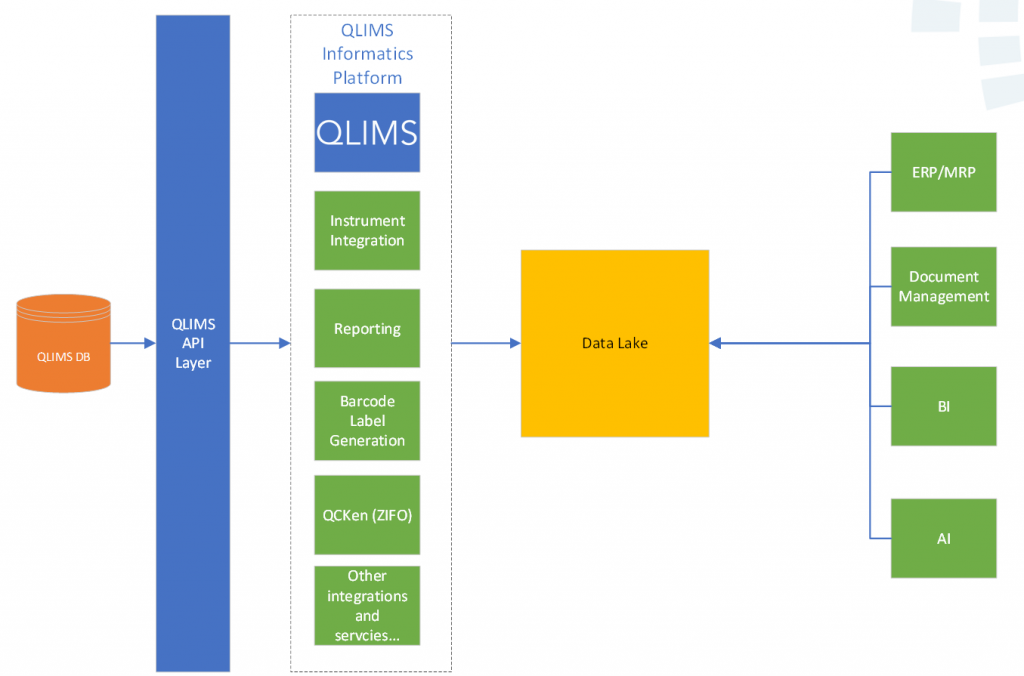
QLIMS as a Platform (3) – Bridge Integration Engine
Have you wanted a cost effective, secure, and flexible way to integrate laboratory instruments and other systems?
The QLIMS Bridge is exactly that and for GMP certified laboratories it provides a streamlined way for validation.
What is the Bridge?
The QLIMS Bridge is a powerful service which monitors folders on a server/computer, then automatically and securely transfers files and maps their associated data to the QLIMS in the cloud.
Running in parralell to the core LIMS it will process large volumes of data from multiple sources in near real time without impacting performance of the LIMS. All the while logging and reporting successes and exceptions.
It is vendor agnostic and very flexible so can be used with a wide range of instruments, file formats and data structures.
What do customers use it for?
- Laboratory instruments – ICP, GC-MS, LC-MS, analysers, micro-plate readers, HPLC and many many more from all the usual vendors plus some you may not know!
- Sample Registration from external collaborators – Many external collaborators will send files with their sample data. Upload this information and automatically register the samples with their metadata.
- Third party portals – Where a company has a web portal which cannot integrate via API the automatic transfer of files to register jobs is made easy
- Reference laboratories – Automated interfacing with reference/contract laboratories to track chain of custody and receive results for work performed and attach reports.
Conclusion
The QLIMS Bridge is another service on the QLIMS platform which brings laboratories a step closer to being fully automated. In line with Lab 4.0 it connects the physical workplace to the digital allowing laboratory staff to focus on value add work. Traditional integrations simply reduce manual data input which is important, but the true power of the Bridge is that from there it can then trigger further business logic.
Contact us today to discuss how it can help.

modestas-urbonas-vj_9l20fzj0-unsplash

 Are there differences in API’s?
Are there differences in API’s?