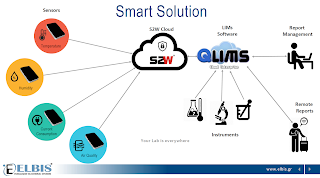
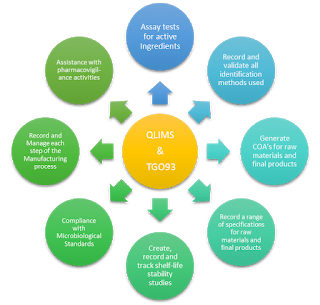
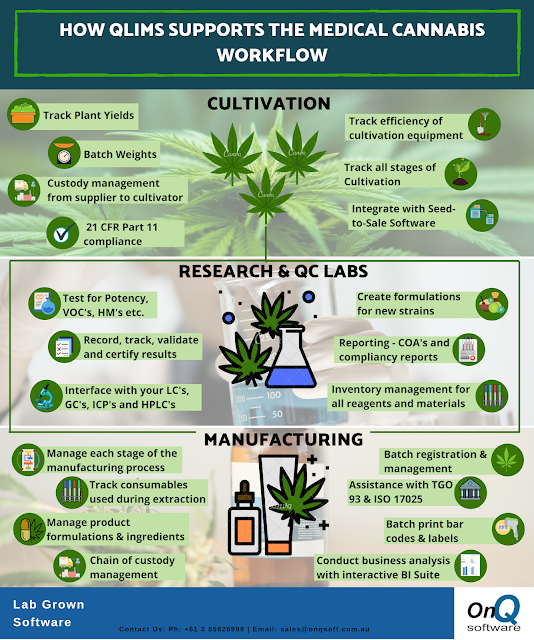
We are pleased to announce that renowned solution provider of the scientific sector, Target Analysis, will be adding QLIMS to their product portfolio and be our new sales distributor in Greece.
We are pleased to announce that renowned solution provider of the scientific sector, Target Analysis, will be adding QLIMS to their product portfolio and be our new sales distributor in Greece.
Adding QLIMS to their portfolio fits in with their vision to provide quality services that will highlight Target Analysis SA as a Total Solution Provider for the supply and after sales support of scientific equipment and software.
Their product range includes: pre-owned systems, analytical instruments, consumables & spare parts, laboratory equipment, software, solvents & chemicals, by a wide network of internationally recognized manufacturers.
In conjunction with ELBIS’s establishment of the QLIMS support centre in Thessaloniki, this partnership now puts Greek laboratories in the unique position of having local access to:
- a highly trained sales team – Target Analysis
- a quality LIMS solution available in Greek – QLIMS
- local support and technical service – ELBIS
——————————-For the Greek version see below————————–
Η Target Analysis είναι ο νέος διανομέας QLIMS στην Ελλάδα
Είμαστε στην ευχάριστη θέση να ανακοινώσουμε ότι ο γνωστός προμηθευτής λύσεων του επιστημονικού κλάδου, η Target Analysis, θα προσθέσει το QLIMS στο χαρτοφυλάκιο προϊόντων και θα είναι ο νέος διανομέας πωλήσεων στην Ελλάδα.
Η προσθήκη του QLIMS στο χαρτοφυλάκιό τους ταιριάζει με το όραμά τους να προσφέρουν ποιοτικές υπηρεσίες που θα αναδείξουν την Target Analysis SA ως Παροχέα Συνολικής Λύσης για την παροχή και υποστήριξη μετά την πώληση επιστημονικού εξοπλισμού και λογισμικού.
Η γκάμα των προϊόντων της περιλαμβάνει: προ-ιδιόκτητα συστήματα, αναλυτικά όργανα, αναλώσιμα και ανταλλακτικά, εργαστηριακό εξοπλισμό, λογισμικό, διαλύτες & χημικά, από ένα ευρύ δίκτυο διεθνώς αναγνωρισμένων κατασκευαστών.
Σε συνδυασμό με τη δημιουργία του κέντρου υποστήριξης QLIMS της Θεσσαλονίκης από την ELBIS, η συνεργασία αυτή θέτει τώρα τα ελληνικά εργαστήρια στη μοναδική θέση της τοπικής πρόσβασης σε:
- μια εξαιρετική καταρτισμένη ομάδα πωλήσεων – Target Analysis
- μια λύση ποιότητας LIMS διαθέσιμη στα Ελληνικά – QLIMS
- τοπική υποστήριξη και τεχνική εξυπηρέτηση – ELBIS
Learn More


 We are pleased to announce that renowned solution provider of the scientific sector,
We are pleased to announce that renowned solution provider of the scientific sector,